Air Pollution
| Environmental Science | |
|---|---|
| Topics | What is the Environment | Population | Ecological Footprint, Food, and Urbanization | Ecology - Definitions and Outline | Energy Flow in Ecosystems | Population and Community Ecology | Biogeochemical Cycles | Biodiversity | Energy | Atmosphere and Climate | Global Warming | Air Quality | Water Quantity | Water Quality | Solid Waste |
Note: Global warming is in its own section |
| To understand the structure of the atmosphere see the section Atmosphere |
Contents
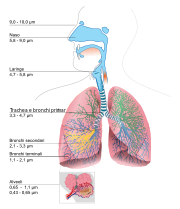
The Atmosphere
The description of the Earth's Atmosphere is now in its own section: Atmosphere
Air pollution
Definitons
- Air pollution
- Presence of chemical(s) in the atmosphere in sufficient quantity to cause harm to humans, other organisms, or materials
- Air pollutants
- Chemicals which cause air pollution
Types
- Primary air pollutants
- Pollutants emitted directly
- Secondary air pollutants
- pollutants created from reactions between primary air pollutant and other chemicals in the air
Standards
- Air Quality Standards
- maximum allowed concentration of a pollutant in the air
- Emission Standards
- maximum amount of a pollutant allowed to be released into the air from a given source
- Criteria air pollutants
- 6 pollutants regulated by the US Environmental Protection Agency (USEPA) using air quality standards
- carbon monoxide
- particulate matter
- sulfur dioxide
- nitrogen dioxide
- ozone
- lead
Major air pollutants
Primary pollutants
- Sulfur dioxide
- Nitrogen dioxide
- Particulate matter and aerosols
- Carbon monoxide
- Lead
- Mercury
- Hydrocarbons
- Chlorinated solvents
- Bezene
- Polynuclear aromatic hydrocarbons
- Asbestos
- Tar/Tobacco smoke
- Formaldehyde
- Radon
- Dioxins
- Pesticides
Secondary pollutants
Other
Sulfur dioxide and acid deposition
Sulfur dioxide - sources
- burning of sulfur-containing compounds including coal and oil
- S + O2 → SO2
- smelting of metal ores
- Cu2S (ore) + O2 → 2Cu + SO2
- Pulp and paper production
- Sulfuric acid manufacturing
Direct effect of sulfur dioxide
- Absorbed by moist respiratory tissue causing restriction of airway
- - especially severe in people with asthma
Acid deposition
Sulfur Dioxide can combine with water in the atmosphere to form sulfuric acid:
- 2SO2 + O2 + 2H2O → H2SO4
Sulfuric acid can removed from the air by rain ("acid rain") or by attaching to dust particles ("dry deposition")
Effects of acid deposition
- Destruction of concrete and stone structures
- The sulfuric acid converts calcium carbonate to calcium sulfate which is more soluble: CaCO3 + H2SO4 → CaSO4 + 2H+ + CO32+
- Acidification of forests, rivers, and lakes
- Some lakes have natural buffering, but many do not.
- Acidified water causes death of fish and other organisms, due to both the lower pH directly and the leaching of metal, especially aluminum. It also causes damage to trees and other plants.
Nitrogen dioxide, ozone, and smog
Nitrogen oxides
Nitrogen can react with oxygen to form nitrogen oxides
- N2O (nitrous oxide) - not toxic but important for global warming
- NO (nitric oxide) - important precursor of ozone and smog
- NO2 (nitrogen dioxide) - important primary pollutant
- N2O3, N2O4, N2O5 - these are very rare in the atmosphere
NO and NO2 are together known as NOx (read as nox)
Nitrogen dioxide
At low temperature combustion nitrogen does not combine with oxygen:
- CH4 + O2 + N2 → CO2 + H2O + N2
However; at high temperatures, such as in a burner or internal combustion engine, Nitric oxide (NO) and Nitrogen dioxide (NO2) are formed [1]:
- CH4 + O2 + N2 → CO2 + H2O + NO
- NO + O2 → NO2
About half the second reaction occurs in the burner/engine, the rest occurs in the air
Nitrogen dioxide - health effects
- lung irritation
- increases susceptibility to lung disease
Ozone
Nitrogen dioxide reacts with sunlight to form nitric oxide and atomic oxygen. This oxygen reacts quickly with molecular oxygen (O2) to form ozone. Ozone can then react with nitric oxide to regenerate nitrogen dioxide.
NO2 + hν → NO + O
O + O2 → O3
O3 + NO → NO2 + O2
Note The ozone formed here is tropospheric ozone ("bad ozone"), not to be confused with stratospheric ozone ("good ozone")
Ozone - effects
Health Effects:
- lung problems
- eye irritation
Environmental Effects:
- damage to plants and trees
Photochemical smog
Ozone is extremely reactive and reacts with any organic chemicals in the air. The result of this reaction between tropospheric ozone and organic chemicals is a "toxic soup" called photochemical smog
Photochemical smog includes NOx, ozone, peroxyacyl nitrates (PANs), aldehydes, and other organic chemicals.
Thermal inversions
Under certain conditions a layer of warm air can occur above cold air. This causes any pollutants to be trapped and not dispersed.
A good animation of thermal inversion can be found here.
Particulate matter (PM) and aerosols
Definition
- Particulate Matter
- Small solid and liquid particles which remain suspended in the atmosphere.
Particulate matter can be divided into two size ranges: 10 - 2.5 μm (PM10) and < 2.5 μm (PM2.5).
Sources
- dust from construction, agriculture, and roads
- forest fires
- burning of solid and liquids -- especially from coal, wood, and diesel fuel
- clearing of land for agriculture
Effects
- The small particles can get trapped in the lining of the lung causing irritation, inflammation, or cancer
- PM2.5 is much more dangerous as it gets deeper into the lung.
Examples:
- Inflammation
- Silicosis (silica dust)
- Black Lung disease (coal dust)
- Cancer
- Asbestos
- Polynuclear aromatic hydrocarbons (PAH)
- Tar from incomplete combustion and tobacco smoke
Toxic air pollutants
Carbon monoxide (CO)
Source:
- complete combustion (burning) of hydrocarbons gives carbon dioxide[2]:
- CH4 + 2O2 → CO2 + 2H2O
- incomplete combustion of hydrocarbons gives carbon monoxide:
- CH4 + 1.5O2 → CO + 2H2O
Effects:
Carbon monoxide binds with hemoglobin in the blood to form carboxyhemoglobin. This means the blood cannot carry oxygen to the cells.
The physical effects include lightheadness, headache, confusion, and dizziness to unconsciousness and death
Lead
Sources:
- paint
- smelters
- batteries
- leaded gasoline
Effects:
- memory loss
- learning difficulties
- nervous system damage
- damage to bones and kidneys
- accumulative poison
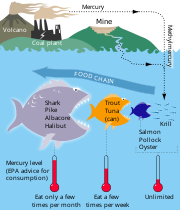
Mercury
Sources:
- burning of coal
- small-scale gold mining
Effects:
- mental effects ("mad-hatter's" disease)
- kidney disease
- accumulative poison
- biomagification - especially in fish (see diagram below)
Volatile organic compounds (VOC's)
Definition:
- Organic compounds which rapidly evaporate.
Examples:
- benzene
- acetone
- solvents
- formaladehyde (from building materials)
Effects:
varies widely depending on substance
- eye, nose, and throat irritation
- dizziness and headaches
- damage to liver, kidney, and nervous system
- actual toxicity varies widely
Indoor air pollution
Indoor air pollution is air pollutants found in your home, office, etc.
Examples:
- formaldehdye
- chlorinated solvents
- pesticides
- tobacco smoke
- radon
Radon
Source:
- Naturally radioactive gas. A decay product of radium (which is a decay product of uranium). Uranium and radium can be found naturally in some rocks especially granite. Radon can then enter houses built on such rocks.
Effects:
- lung cancer (due to alpha radiation)
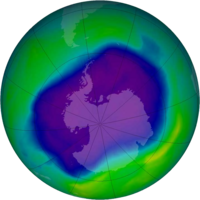
Ozone depletion
Mechanism
As stated above, the stratosphere contains a layer of ozone which protects the Earth from dangerous ultraviolet radiation.
Chlorinated and brominated hydrocarbons can travel unreacted up to the stratosphere. The most important of these are the chlorofluorocarbons (CFC's).
CCl3F + hν → CCl2F + Cl
This free chlorine then reacts with the ozone.
Cl + O3 → ClO + O2
ClO + O → Cl + O2
net O3 + O → O2 + O2
This depletes the amount of ozone present.
Ozone depleting chemcials (ODC)
- chlorofluorocarbons (CFC's)
- hydrochlorofluorocarbons (HCFC's)
- bromochloroflurocarbons (Halons)
- methyl bromide, carbon tetrachloride, methl chloroform
Montreal Protocol
In the 1980's, it was found the ozone layer was thinning. In 1987 a treaty called the Montreal Protocol was signed.
It banned the use of ozone depleting chemicals. As of 2012, 98% of ozone-depleting chemicals were phased out. All countries are in compliance. The ozone layer has stabilized and concentrations of ozone in the stratosphere are now increasing.
| Print this form listing different air pollutants. Then research their effects and fill out the blanks on the form. Be sure to include references
|