Equivalence of Second Law Statements
| Thermodynamics | |
|---|---|
| Introduction | What is this thing called Thermodynamics??? | Definitions | Thermal Equilibrium and Zeroth Law | Limitations |
| First Law | Work, Heat, Energy, and the First Law | Work, Heat, Energy, and the First Law (simplied) | Derivatives | Derivatives Exercise | Reversibility, Enthalpy, and Heat Capacity |
| Second Law | Things to Think About | Observations and Second Law of Thermodynamics | Alternative Approach - the Clausis Inequality | Consequences of the Second Law | Consequences of the Second Law (simplified) | Carnot Principle - motivation and examples | Equivalence of Second Law Statements* |
| Third Law | Third Law of Thermodynamics | Consequences of Third Law* |
| Development of Thermodynamics | The Thermodynamic Network | Network Exercise | Equations of State (EOS) | EOS Example, Reading Tables, and Numerical Analysis | EOS Exercises | Thermochemistry |
* Optional Section | |
This section is Optional.
| There are a number of ways to state the second law. This section first shows how the different statements so far presented are actually equivalent statements. It then presents some additional statements that have been proposed. |
Contents
Equivalence of Second Law definitions
We defined the Second Law of Thermodynamics as:
- With no external input, a system will tend toward disorder
- No system can be developed which completely converts heat to work
These two parts are actually two independent statements. The second statement is often called the Planck statement or the Kelvin-Planck statement. We now wish to show that these are in fact equivalent statements.
To start with we need the Clausis Inequality:
[math]dS \geq \frac{dQ}{T}[/math]
To convert heat to work we must remove heat from the surroundings; therefore, the heat removed must be positive. We also previously noted that temperature is always positive. Then, by the Clausis inequality the change in entropy (S), must also be positive. Finally, we defined entropy as a measure of disorder. Therefore, the disorder of the system increases, which is the same as the first statement.
Clausis statement and equivalence to Planck statement
Previously we mentioned that the second law can be restated as the following:
Heat cannot be moved from a colder region to a hotter region without doing work.
This is known as the Clausis statement. We wish to show this is the same as the Planck statement.
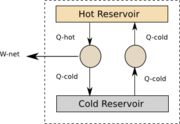
We will begin by considering a system which can move heat from a cold region to a hot region without doing work[nb 1]. Look at Figure 1.
Say the amount of heat removed from the cold region is Qcold. Since no work is being done the amount of heat added to the hot region must also be Qcold. Now consider another heat engine removing heat from the hot region to the cold region. Say it removes the amount Qhot from the hot region, producing Wnet work and returning Qcold to the cold region. If we now look at the net system we see that Wnet = Qhot - Qcold. Now look at the total system. We see that the no heat is leaving the system, but work is. This violates the Planck statement.
Other Statements
Here we will present some other statements of the second law. We will only present them with brief comments.
Caratheodory
Caratheodory developed a very abstract and mathematical approach to thermodynamics. His statement of the second law is:
In the neighborhood of a given state there are states that cannot be reached from the given state by any adiabatic transformation.
This statement has the advantage that it does not require reference to machines. But due to its complex nature it is not used much.
Hatsopoulos-Keenan Statement
Any system having certain specified constraints and having an upper bound in volume can reach from any initial state a stable equilibrium state with no effect on the environment.
Callen's Postulates
One of the most interesting statement of the laws of thermodynamics is that by Herbert Callen[1]. He has used the postulate approach as used in other areas of physics. His four postulates are:
- There exist particular states (called equilibrium states) of simple systems that, macroscopically, are characterized completely by the internal energy U, the volume V, and the mole numbers N1, N2, ..., Nr of the chemical components.
- There exists a function (called the entropy S) of the extensive parameters of any composite system, defined for all equilibrium states and having the following property: The values assumed by the extensive parameters in the absence of an internal constraint are those that maximize the entropy over the manifold of constrained equilibrium states.
- The entropy of a composite system is additive over the constituent subsystems. The entropy is continuous and differentiable and is a monotonically increasing function of the energy.
- The entropy of any system vanishes in the state for which [math]\left (\frac{\partial U}{\partial S} \right )_{V,N_1,...,N_r}=0[/math] (that is, at the zero of temperature).
The first three postulates include the first and second law of thermodynamics. The fourth postulate is the third law of thermodynamics.
Note
- ↑ Here we are using the fact the if A, then B is the same as if not B, then not A.
Reference
- ↑ Callen, Herbert B. (1985) "Thermodynamics and an Introduction to Thermostatistics", John Wiley and Sons