Chemical Equilibria
Chemical Equilibria|Le Chatelier's Principle|Factors Affecting Chemical equilibria| The Haber Process|The Contact Process|Equilibrium Constants
An introduction to Chemical Equilibria
Reversible and Non reversible reactions
Many of the reactions which are generally studied are what are termed as non reversible reactions. Those are the kind during which reactants give products and the products do not decompose to give back the reactants. The reactions go in one direction only. Since the product is not left in contact with the reactant, the reaction is irreversible.
Example:
I2(s) --> I2(g)
However, under different conditions, this reaction becomes a reversible one. When the system is closed, the product can get back to the reactant.
Example:
I2(s) --> I2(g)
- Note: During a reversible reaction, no components leave or enter the system.
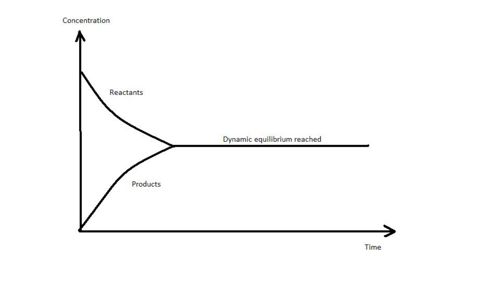
Dynamic Equilibrium
When an equilibrium is established, the concentrations of the reactants and products remain constant as long as external conditions are not changed.
However, the reaction has not stopped. The rate at which I2(g) is formed is exactly same as the rate at which I2(s) is formed. As a result, it seems that the reaction is no longer continuing because we no longer see any formation of more black I2(s) particles or purple I2(g) vapours . We could have concluded that the reaction taking place is static but it is also known that particles are in constant random movement. Thus it seems more likely that the particles are continuously moving which leads to a system known to be in a Dynamic Equilibrium.
Static Dynamic
The Dynamic equlibrium of a system is a state during which the rate of the forward reaction is equal to the rate of the backward reaction.